Time
additional production time per year of FBD
Quality
control – of finished tablets and capsules
Regulation
ATEX, 21 CFR Part 11 IQ/OQ conformity
Measurably better - No matter whether granulation or spray drying processes, tablets, capsules or micro dosing, our high-performance solutions are simple: we provide patented microwave technique tied to passion driven services. Why? The answer is as simple as our solutions: to improve the production process of our clients. Or, in other words: to help our clients to achieve better results.
Our solutions have been developed and designed hand-in-hand together with the pharmaceutical industry and, therefore, fulfill the requirements of 21 CFR Part 11 which are available in different Atex-standards including IQ/OQ-documentation.
The TEWS method enables pharmaceutical companies to gain high-precision data about the level of moisture content and density of their products. It’s a one-stop-shop without any sample preparation to optimize the whole production process in terms of cost savings and quality control.
They trust us
Water Matters.
Product quality and -efficiency can be significantly optimized by controlling the moisture content in almost all process steps. Managing the moisture of pharmaceutical products is a key factor to achieve better results. TEWS patented solutions deliver high precision results. It's what matters when it comes to company goals and how to achieve them faster.
- Production Efficiency – Gaining up to 300 % additional production time of FBD per year.
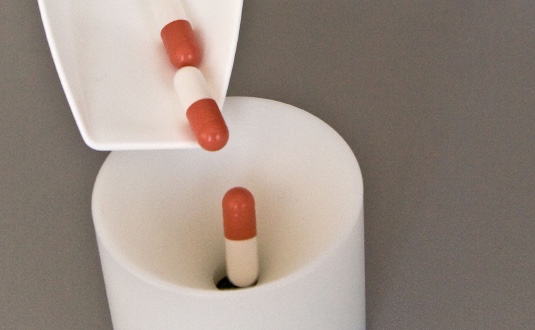
- Product Quality and Product Safety – A precise moisture level is decisive for the quality. Especially in the pharmaceutical industry a wrong moisture level can even harm the product safety as active ingredients may change and turn into toxic.
- Securing High-Speed-Production – Modern high-speed machines are fragile in a sense that minor deviations from the specified inflow or any piling-up within the production flow leads to severe interruptions. With up to 40,000 measurements per second we are well ahead of all pharmaceutical-related production processes.
- Plant Efficiency – With our measurement results operators can utilize their advanced machines at an optimum and reduce downtimes and waste to a minimum.
- Storage Efficiency – Precise compliance with the recommended water content of components secures product integrity.
It Takes Two.
TEWS patented two-parameter measurement solutions enable pharmaceutical companies to work with high precise data in both ways at the same time. Data that relate to the moisture content of pharmaceutical products as well as their density. Knowing the precise weight of pharmaceutical products during the manufacturing process leads to even better results in terms of product quality and -efficiency.
- Mass Flow and Balance Check – Accurate density information being used to assure constant filling levels.
- Quality Control – Securing homogenity of your products.
- Product Safety – up to 100% control of finished tablets and capsules to ensure right target weight and content at high speed.
- Plant Efficiency – Automatic dosing at high-speed without balance checks.
Areas of Application.
Learn More About Our Patented Solutions.
How Can We Help You?